PH Defination, Calculation, Limitation
In this blog how to learn PH Defination calculation limitation. Soren Peter Lauritz Sorensen was denish chemist known for the introduction of the concept of PH scale measure acidity and basicity.
Expand Your Knowledge on [Glass Lined Reactor]
What is full form PH ?
PH full form is potential of hydrogen.
PH defined as the negative logarithm of H+ ion and H3O+ concentration.
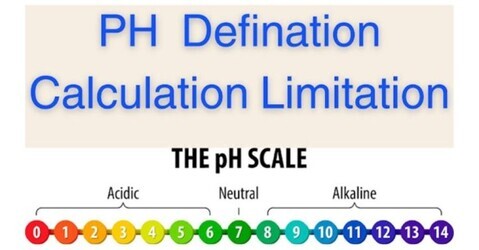
PH scale is measuring Acid and Bases.
What is a PH scale ?
PH scale range is 0 – 14
Acidic PH = 0 – 7
neutral PH = 7
Alkaline PH = 7 – 14
Example
| NO. | Materials | PH Value |
| 01 | Battery Acid | 0 |
| 02 | HCL | 1 |
| 03 | Lemon Juice | 2 |
| 04 | Vinegar | 3 |
| 05 | Orange Juice | 3.5 |
| 06 | Coffee | 5 |
| 07 | Pure Water | 7 |
| 08 | Human Blood | 7.35 |
| 09 | Milk | 6.5-6.7 |
| 10 | Backing Soda | 8.5-9.0 |
| 11 | Ammonia Solution | 11 |
| 12 | Bleaching liquid | 12.6 |
| 13 | Oven Cleaner | 13 |
| 14 | NaOH | 14 |
PH Formula
PH = -log [H+]
PH = -log [H3O+]
POH = -log [OH–]
Distilled waterH3O+= OH– = 10 -7 at 298 K.
If concentration of H3O+ is more than 10 -7 then solution becomes Acidic.
If concentration of OH– is more than 10 -7 then solution becomes Basic.
Distilled water concentration of H3O+ and OH– is a same so it is neutral.
Why PH value is 0 to 14 ?
H3O+= OH– = 10 -7
=H3O+ × OH–
= 10 -7 × 10 -7
= -log10 [H3O+] + -log10 [OH–]
= -log10 [ 10 -7] + -log10 [ 10 -7]
= -log10 10 -14
= 14 Log10 10 p[OH–] + p[H3O+]
= 14
PH measuring 3 way
01.) Litmus test

PH Measuring through litmus paper :
Basic :
RED ——- BLUE
Acidic:
BLUE ——- RED
02.) universal indicator
Universal indicator is dropwise feed in solution then colour change according PH.
Acidic colour red to pink.
Basic color gives to blue.
03.)PH meter
PH meter electrode use potassium chloride solution based KCL is PH neutral.
PH meter is device by we can check accurate PH of solution.
How to calculate PH ?
01.) Define 0.01 M HCL PH ?
HCL —— H+ + Cl–
Initial
0.01 0.0 0.0
Final
0.0 0.01 0.01
= 10 -2
PH = -log [H+]
PH = -log 10 -2
PH = 2 log 10
PH =2
02.) Define 0.005 M H2SO4 PH ?
H2SO4 —– 2H+ + SO4-2
0.005 0.0 0.0
0.0 0.005 0.005
0.0 0.005 × 2 Mole 0.005
0.0 0.01 0.005
= 10 -2
PH = -log [H+]
PH = -log 10 -2
PH = 2 log 10
PH =2
03.) Define 0.01M NaOH PH ?
NaOH —— Na+ + OH–
0.01 0.0 0.0
0.0 0.01 0.01
= 10 -2
POH = -log [OH–]
POH = -log 10 -2
POH = 2 log 10
POH = 2
PH + POH = 14
PH = 14- 2
PH = 14 – 2
PH = 12
Why doesn’t solvent have a PH ?
Most organic solvent do not have ionizable H+ ions so they won’t give a proper reading in a PH meter.
PH Scale Limitation ?
PH scale on for Aqueous solution.
PH applicable for concentration H3O+ ions is less than 1M.
PH limit is 0 – 14.