Carbon Disulfide
What is Carbon Disulfide (CS2) ?
- Carbon disulfide (CS₂) was first discovered and synthesized by the German chemist Wilhelm August Lampadius in 1796.
- Carbon Disulfide is an inorganic compound with chemical formula CS2.
- It is a colorless, volatile, and flammable liquid with a strong, characteristic odor.
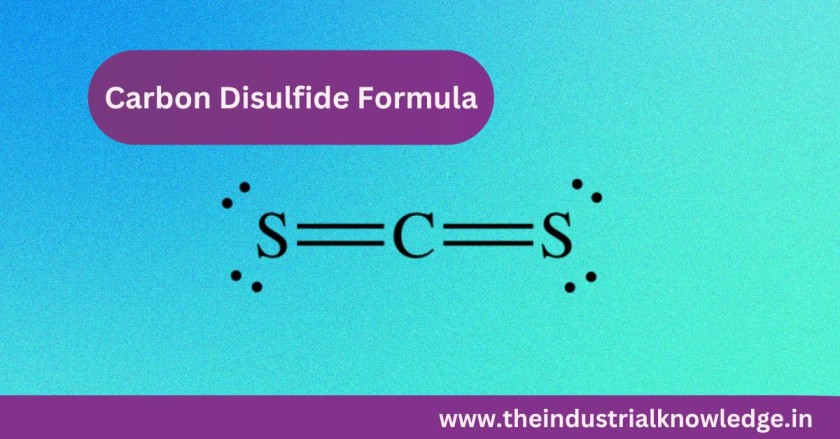
- CS2 is simple and composed of a single carbon atom double-bonded to two sulfur atoms (C=S=C). The linear order of the compound leads to CS₂ being classified as a non-polar molecule, and that is what makes it an effective solvent.
Carbon Disulfide Storage Specific Recommendations for Installation :
CS2 storage vessels should be fitted with two independent continuously level monitoring instruments of which one should be provided with high level alarm.
Properly designed rupture discs should be used instead of asbestos plates for the Cs2 manufacturing furnaces.
Safety seal outlet of the CS2 furnace should be extended above the roof level.
U/S 2(d) of the PLI Act, a schedule is notified of hazardous substances. Liability of PLI policy arises if hazardous substances are handled in quantity equal to or more than tins threshold quantity. CS2 threshold quantity is 20 tons.
Carbon disulfide synonyms :
Carbon Bisulfide, Disulfido Carbon, Methane Dithione
Carbon Disulfide Formula :
Carbon Disulfide Physical & Chemical Properties :
CAS No : 75-15-0
Molecular formula : CS2
Appearance : Clear, colorless to pale yellow liquid
Molecular weight : 76.1
Odor : Strong, disagreeable odor
Specific Gravity : 1.26 g/mL @ 20°C
Vapor Density (air=1) 2.63
Solubility : Slightly soluble in water
Melting Point -111 °C
Boiling Point : 46°C
Autoignition Temp.: 90°C (194°F)
Flash Point: -30°C (-22°F) CC
Evaporation rate : 22.6 (Butyl acetate = 1)
Vapor Pressure (20°C) 300 mm Hg
LEL : 1.3%
UEL : 50 %
Carbon Disulfide Process Reaction :
The production of carbon disulfide (CS₂) involves the reaction of carbon (usually in the form of coke or charcoal) with sulfur at high temperatures.
Coke, charcoal, or another form of carbon react with sulfur in furnace at 800°C to 1000°C (1472°F to 1832°F).
C+2S→CS2
The reaction is endothermic, meaning it requires continuous heat input to maintain the necessary temperature.
Carbon Disulfide Reactions :
- CS2 react with oxygen produce CO2 (carbon dioxide) and SO2 (sulphur dioxide).
CS2 + 3 O2 → CO2 + 2 SO2
- CS2 react with Halogen produce CCL4 (carbon tetrachloride) and S2Cl2 (sulphur dichloride).
CS2+3Cl2→CCl4+S2Cl2
- CS2 react with alkali hydroxide produce alkali thiocarbonates(carbon tetrachloride) and H2S (hydrogen sulfide gas).
CS2+2NaOH→Na2CS3+H2S
CS2+2KOH→K2CS3+H2S
- CS2 react with hydrogen produce CH4 (methane) and H2S (hydrogen sulfide gas).
CS2+4H2→CH4+2H2S
CS2 Uses :
- The principal industrial CS2 consuming 75% of the annual in production.
- CS2 widely used in the production of carbon tetrachloride.
- It is widely used in synthesis of organosulfur compound.
- Used as preparing soil disinfectants.
- Used in the manufacturing of viscose rayon and cellophane film.
- Used to manufacture electronic vacuum tubes.
- Used as a solvent in rubber-making industries.
- Used in camphor.
- Used in generating petroleum catalysts.
- Used as pesticide intermediate.
- Use as a solvent for iodine, phosphorous.
-
Carbon disulfide is also used in the synthesis of carbon tetrachloride, thiocarbonates, and thiourea.
-
carbon disulfide is used as a reagent and solvent, especially in infrared spectroscopy due to its transparency to IR radiation in the region of 4000-1000 cm⁻¹.
CS2 Occupational Diseases, Symptoms and Diagnosis :
- BEI full form is biological exposure index.
- 2-thiothiazolidine-4-carboxylic acid (TTCA) is a urinary marker that can indicate exposure to carbon disulfide vapor.
- Indian standard code of safety for CS2 is 5685.
- Schedule No 14 & 22 are mentioned in Gujarat Factories Rule 14 & 22 for CS2.
- Notifiable Diseases under the Factories Act 1948, section no. 89 & 90.
| Diseases caused | Occupational involved | Signs & Symptoms | Diagnosis / Indicator (see BEI if available) |
| Carbon disulfide |
|
|
|
Carbon Disulfide Flammability, Toxicity, Reactivity :
Toxicity :
CS2 TLV value is 10 ppm.
CS2 TWA value is 10 ppm and 30 Mg/m3
Exposure to CS₂ vapors can cause severe health effects, including headache, dizziness, fatigue, and nerve damage. Chronic exposure may lead to serious neurological damage.
CS₂ can be absorbed through the skin, causing irritation or burns.
Flammability :
CS₂ has a low flash point and can easily ignite, forming explosive mixtures with air. It requires strict handling protocols to avoid accidental ignition. The explosive range of CS₂ in the air is between 1.3% and 50% by volume, making it extremely hazardous.
Reactivity :
- Carbon disulfide (CS₂) reacts dangerously with strong oxidizing agents, including peroxides and nitrates, potentially causing explosions.
- It also reacts violently with halogens like chlorine and bromine, which can lead to detonation.
- When exposed to strong bases or alkali metals like sodium and potassium, CS₂ can decompose, releasing toxic and flammable gases such as hydrogen sulfide.
- Concentrated nitric acid poses a significant risk as it can oxidize CS₂, leading to hazardous reactions.
- CS₂ is highly flammable and can ignite easily from heat, open flames, or static electricity, requiring strict safety precautions.
Q & A :
01. Is cs2 a gas or liquid?
Ans : Carbon disulfide (CS₂) is a colourless to light yellow volatile liquid at room temperature, with a boiling point of 46.3°C (115.3°F).
02. What is CS2 used for?
Ans : CS₂ is used as a solvent in the production of cellophane and rayon. It’s also used in the manufacture of carbon tetrachloride, rubber, pesticides, and other chemicals.
03. Is carbon disulfide poisonous?
Ans. Yes, carbon disulfide is toxic. Exposure can cause harmful effects on the nervous system, cardiovascular system, and skin. Prolonged or high-level exposure can be particularly dangerous.
04. Where does carbon disulfide come from?
Ans : Carbon disulfide is primarily produced industrially by reacting carbon (such as coke or charcoal) with sulfur at high temperatures. It can also be found naturally in volcanic emissions and in crude petroleum.
